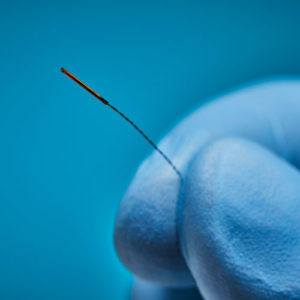
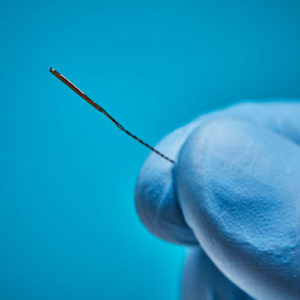
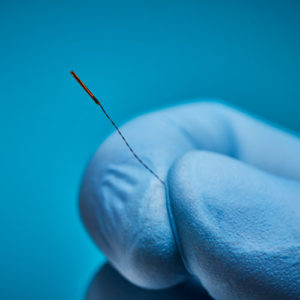
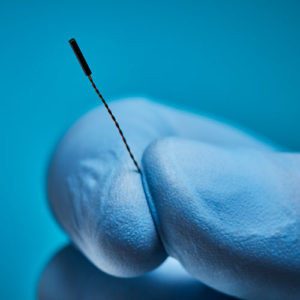
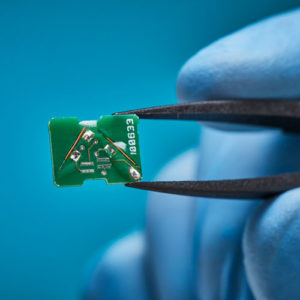
Aurora® Sensors
A full suite of 5DOF and 6DOF sensors for adding EM Navigation to interventional medical devices and minimally invasive procedures.
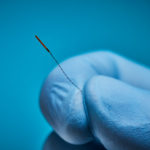
Target, Access, and Reach the Smallest Treatment Areas
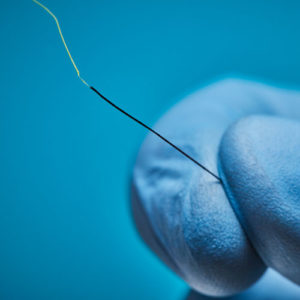
Aurora sensors are a key component of the Aurora electromagnetic (EM) navigation solution, where they locate OEM medical devices such as catheters, scopes, transducers, and guidewires during a procedure. Using the embedded sensor, the device’s position and orientation are shown in real time as it’s navigated inside the body – no line of sight is needed.
Aurora sensors are available in 5DOF or 6DOF (Degrees of Freedom), which refers to the direction of movement reported during navigation. 6DOF reports all movement; 5DOF reports everything except roll. Sensors also have different core types for versatile integration options: solid-core sensors go inside the device, while the device passes through the centre of our proprietary hollow-core sensor.
The breadth of our sensor portfolio (20+ sensors), and the depth of our integration expertise (40+ years), empower our OEM customers to develop new navigated medical devices and new minimally invasive techniques to accurately target, access, and reach the smallest treatment areas.

Navigation without Fluoroscopy
A current is induced inside the sensor, which is converted into a signal that determines the sensor’s (and the device’s) real-time position and orientation in the measurement volume. No fluoroscopy is needed to “see” the sensor.
Versatile Medical Device Integration
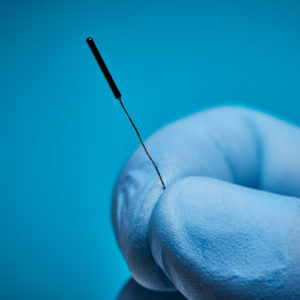
Aurora sensors can be embedded in several ways to best support how, where, and when the device is used during a procedure. For example, a 5DOF sensor could be placed at a needle’s tip to track its location and trajectory.

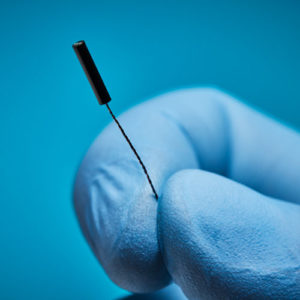
Sensors as Small as ø0.3 x 2.5 mm
This micro Aurora 5DOF sensor measures just ø0.3 x 2.5 mm—the smallest on the market—for integration with microcatheters and small devices for safe navigation through difficult-to-access lesions and vessels.
Optimized Sensor Tracking Performance
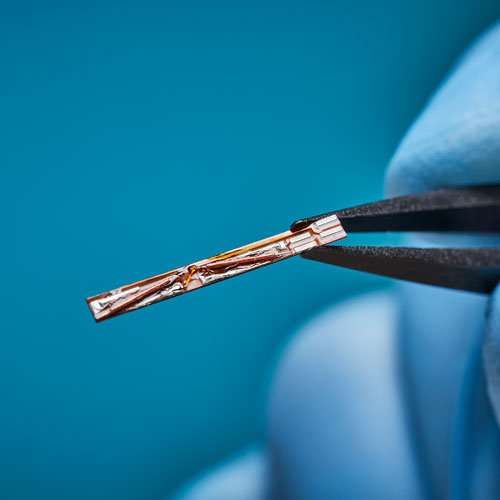
Our proprietary Aurora Commutator Technology improves sensor tracking performance by suppressing signal distortions. Customers can apply this technology to their specific devices and applications to optimize tracking accuracy.

Discover the benefits of going small – navigate smaller vessels with an EM sensor sized for miniaturized interventional devices.
What to Consider when Selecting a Sensor
Sensor attributes are interconnected in terms of integration method, application use, and tracking performance. Our expert NDI Integration Engineers can help you select the right sensor for your medical device and procedure workflow.
A 6DOF sensor reports roll; 5DOF does not. NDI offers Aurora 6DOF and 5DOF sensors.
The sensor’s diameter and length determine its fit inside/outside the medical device.
The point of interest decides the sensor’s location for the device, e.g., at the tip of a needle.
The device passes through a hollow-core sensor; solid-core sensors go inside the device.
Larger sensors have a higher signal for a greater tracking distance in the measurement volume.
Larger sensors are more accurate than smaller sensors.
Up to 32 Aurora 5DOF sensors or 16 6DOF sensors can be tracked in the measurement volume.
Aurora 5DOF Sensors
Aurora 6DOF Sensors


Download the Aurora
Small Sensor Data Sheet
Add real-time targeting and navigation to catheters, without the need for fluoroscopy, with the market’s smallest EM sensor.
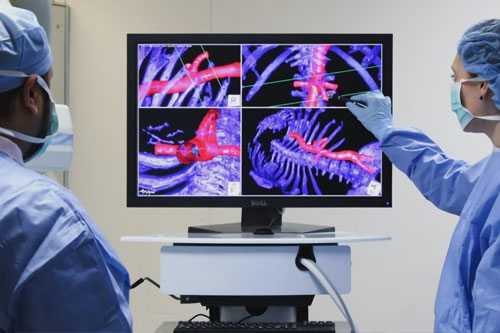
Partner with NDI to Drive Your Next Medical Breakthrough
Explore NDI’s case studies to understand our comprehensive approach to partnerships based on innovative technology and integration expertise.

Download the
EM Education Guide
Download this 12-page guide to learn more about Electromagnetic Navigation Technology and the advantages it can provide to medical device OEMs.