Home > Why Accuracy Matters: Precision in OEM Surgical Navigation
Why Accuracy Matters: Precision in OEM Surgical Navigation
Measurement accuracy and reliability are the foundation of the NDI optical measurement and electromagnetic tracking solutions. However, accuracy represents more than measurement values hitting a theoretical ‘bullseye’ – although that’s important, too. It symbolizes the confidence users feel when they navigate an OEM surgical instrument’s path to a physical target (treatment site) and reach it exactly as expected, every time.
During OEM surgical applications, high measurement accuracy can allow for more precise instrument targeting of very small lesions in areas of the body that would have once been inoperable. Being able to avoid delicate and/or critical anatomy can provide safer access to those treatment areas. As a result, using an accurate OEM surgical system can help shorten procedure times and minimize surgical invasiveness, which can improve patient outcomes. For example, in radiation oncology, high measurement accuracy can reduce the treatment margins when targeting tumours, thereby preserving healthy tissue.
How is Accuracy Determined?
The term accuracy encompasses the concepts of trueness and precision. Trueness refers to the closeness of agreement between the mean of test results and the true (accepted) reference value. Precision refers to the closeness of agreement between test results.
The concepts of trueness and precision can be visually depicted as a target, where:
Related Article:
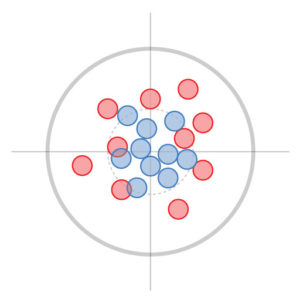
Trueness represents how close measurements are to the bullseye (the true reference value). In this example, the red and blue targets are both true, but the red measurements are less precise because they are far apart.
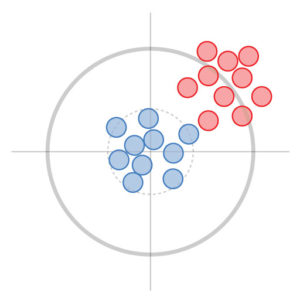
Precision represents how close repeated measurements are to each other (test results). In this example, the red and blue measurements are both precise, but the red measurements are less true because they are off target.
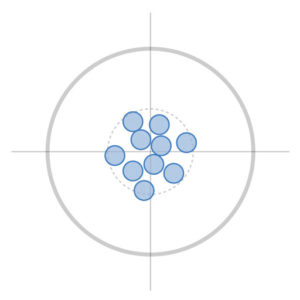
Accuracy represents measurements that are close to the bullseye and close to each other. In this example, as with all NDI optical measurement and electromagnetic tracking solutions, the measurements are both true and precise.
NDI’s optical measurement and electromagnetic tracking solutions adhere to the ISO 5725-4:2020 standard for trueness and precision. Our solutions are certified measurement systems, the outputs of which can be traced back to standards set by the National Institute of Standards and Technology (i.e., NIST traceable).
Measurement You Can Trust®
This focus on trusted measurement performance is evident throughout the design, manufacture, and integration of the Polaris, Aurora®, and 3D Guidance® solutions:
Characterization
Our characterization models are based on proprietary algorithms that leverage decades of progressive applied physics and mathematics research. These models encompass every conceivable aspect of 3D tracking to maximize spatial measurement accuracy.
End-to-End Design
Solution components are designed to work together, with an emphasis on minimizing systematic noise during measurement. Accuracy and repeatability are maintained among the different system components and throughout connection points.
Manufacturing Process
The design and manufacture of Polaris, Aurora and 3D Guidance components and passive spheres adhere to numerous ISO, IEC, UL, CSA, EC and other regulatory and quality standards, providing a reliable tracking system that operates exactly to stated accuracy specifications.
Factory Calibration
The accuracy and repeatability of 3D positions are verified using a coordinate measuring machine (CMM) within our manufacturing facility for validated measurement performance that optimizes trueness and precision. No in-field calibration is required.
OEM Industry Partnerships
Our partners are at the forefront of medical discovery, pushing the boundaries of what surgical navigation systems are capable. Our partners’ unique insights have helped NDI to innovate increasingly more versatile tracking solutions.

Customer Applications
See examples of how medical device OEMs have integrated 3D tracking technology into their system workflow.
Legal Disclaimer
NDI tracking and measurement products are general metrology components that can be integrated into customer products, research experiments, and/or as components of medical devices that require precision measurement and tracking. While NDI components and technology can be integrated into original equipment manufacturer (OEM) medical devices, they are not specifically intended for a given application and, as such, have not been developed or manufactured in accordance with medical device standards. It remains the responsibility of the OEM customer or end-user to determine and test the suitability of NDI components and technology for their intended use, including performing any required ethics approval, verification, and validation required to demonstrate suitability and compliance. System-level testing, certification, and validation are the responsibility of the original equipment manufacturer or the applicable end-user and should be completed prior to the use of NDI products or technologies in any application.